Kinetics
Problem of practice:
Students have a hard time understanding the breaking and forming of bonds through a chemical reaction. They can also get confused by the concept that increasing surface area means decreasing the size of the particles. It is hard for students to conceptualize what is happening at an atomic level.
 Our solution:
Our solution:
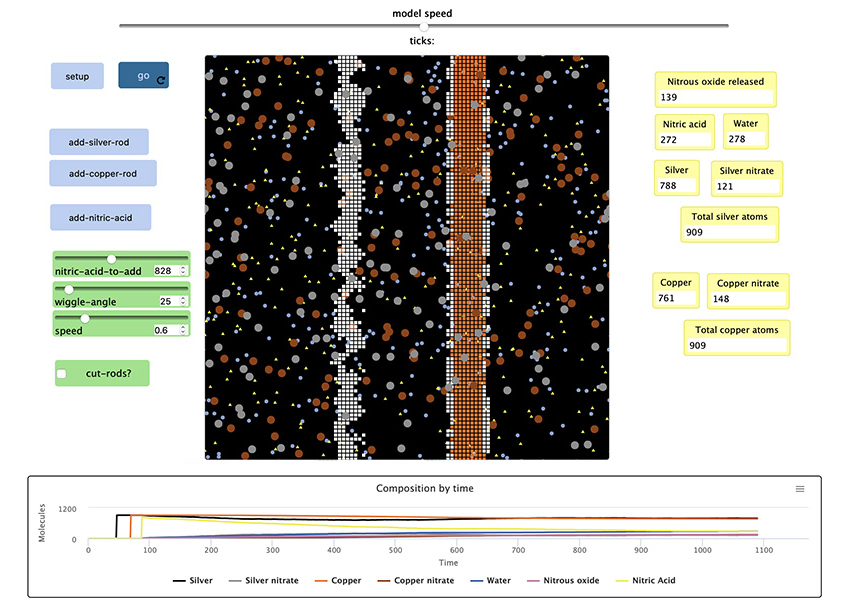
This Chemistry+C activity features a model that simulates how particles interact at the atomic level. The NetLogo model uses common modifications that chemists use to increase the rate of a reaction; it includes an option to increase surface area by cutting up the silver and copper bars while keeping the amount of particles constant. Students can manipulate the temperature by changing the speed of the particles, and the concentration of the beginning nitric acid can be adjusted with a slider value. Additionally, adjusting the wiggle-angle brings stirring into the model.
What students will learn:
Students will learn about four of the modifications to increase the rate of the reaction by using a starter model that does not have these modifications. After making observations of the starter model, students will develop their own ideas to increase the rate of the reaction. A graph and counters are provided so the students can observe the effects of modifications over the same amount of time.
Full Chemistry+C units list:
Introductory Unit: Epidemic: Introduction to Modeling and Simulation
Plus:
- Matter: Physical Change of Salt in Water
- Atomic Structure: Rutherford’s Gold Foil Experiment
- Chemical Reactions: Photosynthesis and Cellular Respiration
- Kinetics: Rate of Reactions
- Titration: Acid-Base Neutralization
- Redox: Electrolysis within a Battery
If you would like access to these Chemistry units, please complete the Science+C Interest Form.